
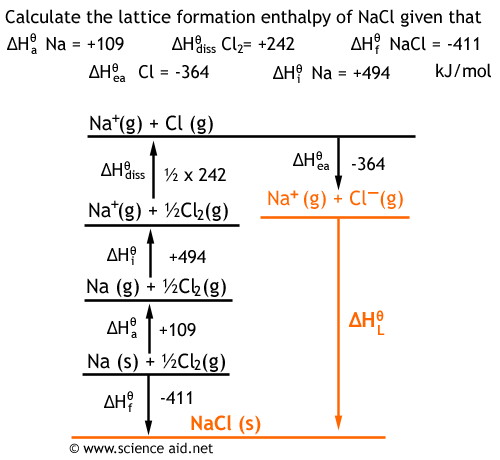
It is calculated by measuring the energy required to break the bonds in the compound and separate the ions, and then subtracting this value from the energy required to form the bonds in the compound. Lattice enthalpy, on the other hand, is a measure of the energy change that occurs when an ionic compound is formed from its component ions. The lattice energy of an ionic compound is therefore the sum of all these energies, and it reflects the overall stability of the compound. The lattice energy of the ionic compound.Formation of the gaseous atoms from the element.ISRO CS Syllabus for Scientist/Engineer Exam Find step-by-step Chemistry solutions and your answer to the following textbook question: The lattice energy of sodium chloride, NaCl, is -787.5 kJ/mol.ISRO CS Original Papers and Official Keys To make the understanding more lucid, we have taken one of the very common crystals, very popular in the crystallographic community, NaCl crystal having 6:6 co.GATE CS Original Papers and Official Keys.DevOps Engineering - Planning to Production.Python Backend Development with Django(Live).
Equation of lattice energy of nacl android#
Equation of lattice energy of nacl full#
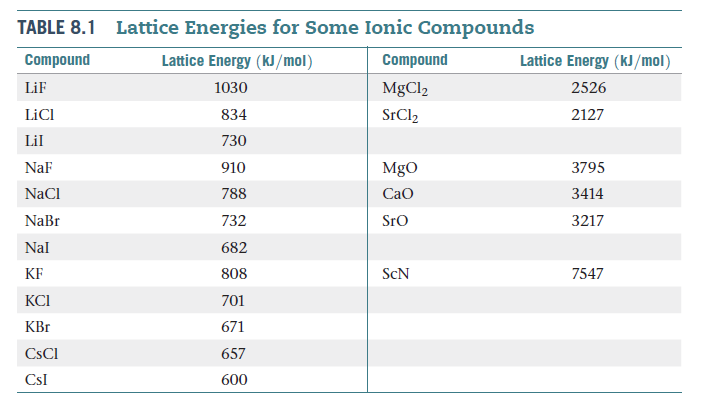
Full Stack Development with React & Node JS(Live) First principles calculations based on DFT have been performed on crystals of halides (X F, Cl, Br and I) of alkali metals (M Li, Na, K, Rb and Cs).Java Programming - Beginner to Advanced.Data Structure & Algorithm-Self Paced(C++/JAVA) NaCl (s) Na+ (g) + Cl (g) Here, the energy that must be supplied to 1 mole of sodium chloride to separate it into gaseous Na + and Cl ions is 786 kilojoules.Data Structures & Algorithms in JavaScript.Data Structure & Algorithm Classes (Live) This Lattice Energy Formula is as follows: U is always a positive number, and it represents the amount of energy required to dissociate 1 mol of an ionic solid into the gaseous ions.


 0 kommentar(er)
0 kommentar(er)
